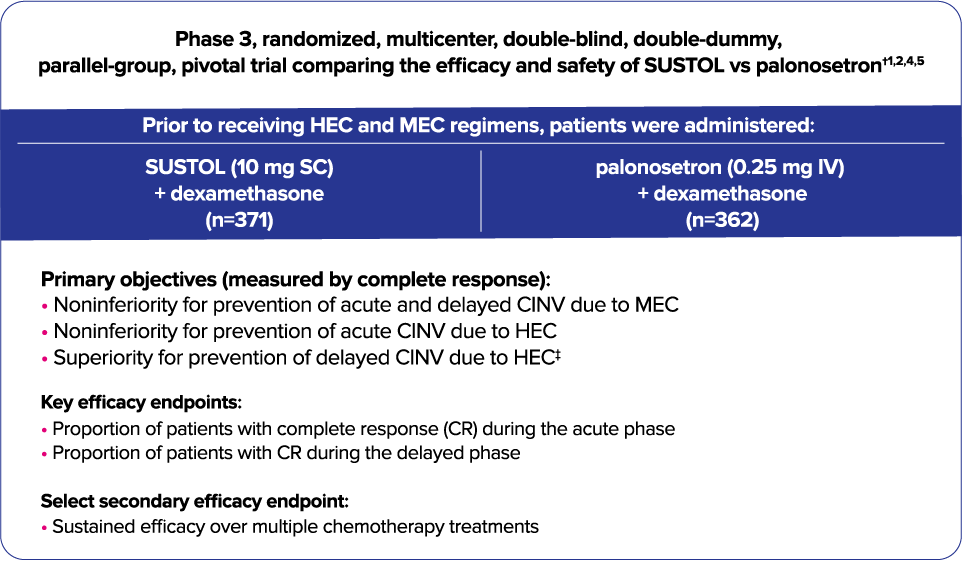
Trial 1—Phase 3 study to assess CINV prevention in HEC and MEC with a 2-drug regimen1,2
- All patients were concomitantly administered intravenous dexamethasone.1
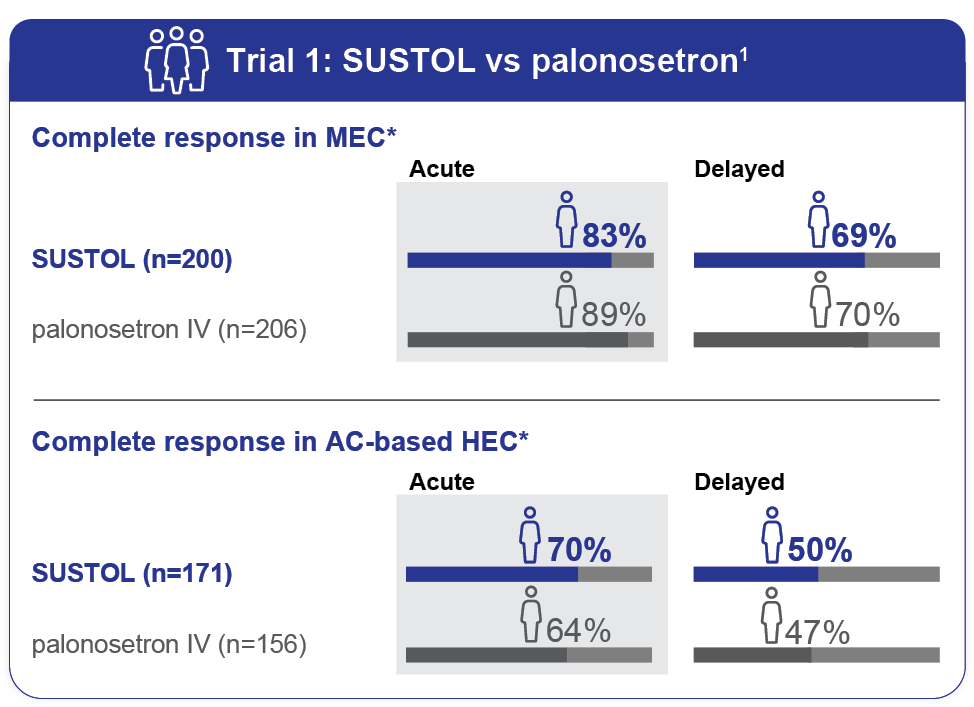
- As measured by the primary endpoint of complete response, SUSTOL was noninferior to palonosetron for control of acute and delayed CINV after MEC and AC-based HEC regimens1
- Trial 1 did not show a significant difference in delayed-phase CINV in HEC; however, there was a trend favoring SUSTOL1
- Because a 2-drug study is not the current recommendation for CINV prevention in patients receiving HEC, as a next step, Heron conducted a 3-drug vs 3-drug superiority trial3
SUSTOL demonstrated full 5-day efficacy in the prevention of acute and delayed CINV1
| † | Due to the number of cisplatin-treated patients in SUSTOL Phase 3 clinical trials (n=358; 26% of HEC-treated patients), efficacy in this population has not been established. SUSTOL is not indicated in patients treated with cisplatin.1,5,6 |
| ‡ | Palonosetron is not approved for the treatment of delayed CINV due to HEC. The endpoint of superiority in the delayed phase following HEC was not achieved.1,7 |
- The most common chemotherapy regimens were carboplatin-based regimens (31%) and AC-based chemotherapy (45%)1
- Complete response was defined as no emetic episodes, including retching, and no use of rescue medications1
AC=anthracycline/cyclophosphamide; CINV=chemotherapy-induced nausea and vomiting; HEC=highly emetogenic chemotherapy; IV=intravenous; MEC=moderately emetogenic chemotherapy; SC=subcutaneous.