SUSTOL—robust safety profile in clinical studies involving >2400 patients1-3

- Most side effects seen with SUSTOL are consistent with what is expected of 5-HT3 RAs4,6-10
- SUSTOL had no significant effect on any electrocardiogram interval, including QTc duration, in clinical trials4
Due to the unique polymer delivery system of SUSTOL, injection site reactions (ISRs) are expected but generally manageable4,5
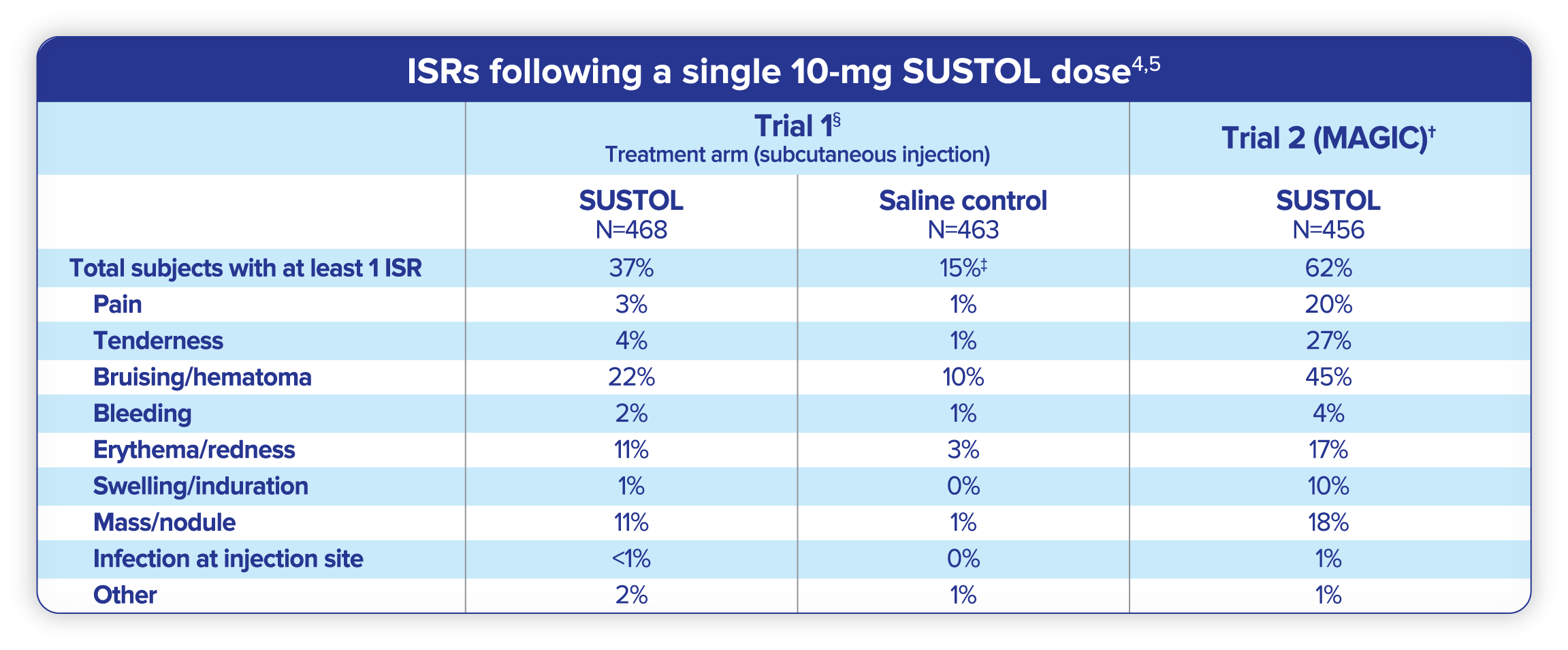
- Three percent of patients receiving SUSTOL reported severe ISRs11
- Fewer than 1% of patients discontinued treatment due to ISRs
- Frequency and severity of most ISRs decreased in subsequent cycles
- All reactions occurring at ≥3% in the SUSTOL group.4
- Modified Absorption of Granisetron In the Prevention of CINV.12
- The placebo subcutaneous injection for Study 1 was normal saline; for Study 2 it was a SUSTOL-matched control (the polymer vehicle without active drug).4
- All groups received dexamethasone.4
5-HT3 RA=5-hydroxytryptamine 3 receptor antagonist; IV=intravenous; QTc=corrected QT interval.