CINV still occurs despite established antiemetic treatment
~50% of patients experience a CINV event despite use of guideline-recommended antiemetic regimens1
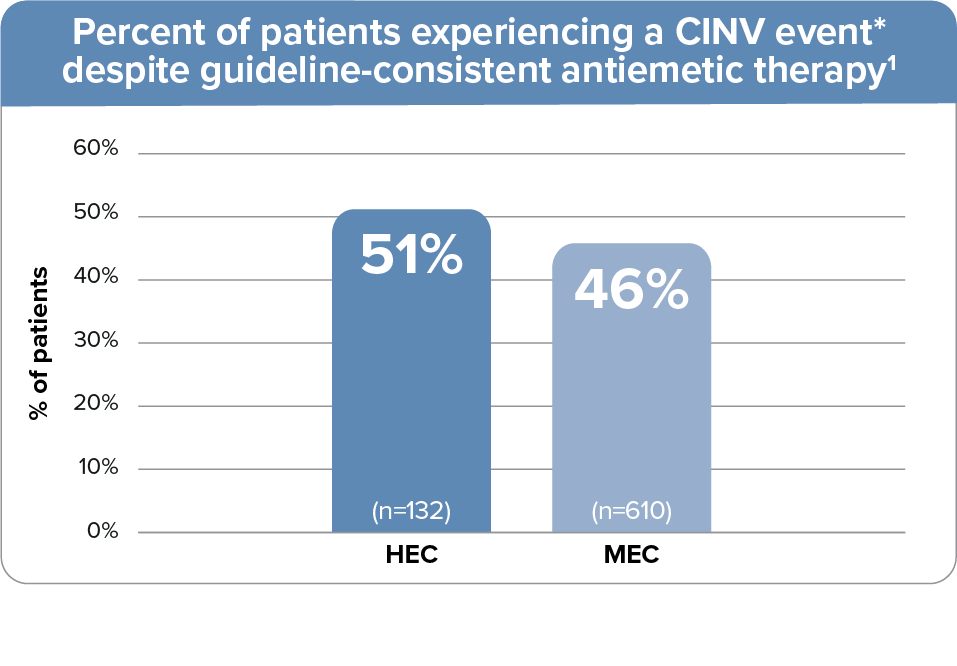
- Data from a prospective observational study where 742 patients received guideline-adherent antiemetic
treatment for single-day HEC or MEC at 4 oncology practice networks, all using EHR systems, in Georgia,
Tennessee, and Florida1
- The guideline-recommended HEC regimen included a 5-HT3 RA + NK1 RA + dexamethasone; the MEC regimen included a 5-HT3 RA + dexamethasone1
- 5-HT3 RA used: palonosetron (94%) or ondansetron (5%)1
- CINV event defined as emesis or clinically significant nausea on days 1-5.1
Data from a Phase 3 trial also show that treatment failure† is common despite the use of prophylactic treatment2

†Treatment failure was defined by first emetic episode or use of rescue medication.2
- Patients experienced high rates of nausea and/or vomiting on days 2 and 32
- Data from a pivotal, Phase 3, double-blind, randomized trial that evaluated the safety and efficacy of palonosetron in the prevention of acute and delayed CINV following HEC. Patients received 0.25 mg or 0.75 mg of palonosetron, or 32 mg of ondansetron
Early control of CINV is important for future efficacy2
- In an analysis of 991 patients receiving chemotherapy, patients with uncontrolled CINV at cycle 1 were almost 4 times more likely to have anticipatory CINV prior to cycle 23
The high rates of treatment failure reveal the need for a 5-HT3 RA that broadly prevents both acute and delayed CINV2-4
5-HT3 RA=5-hydroxytryptamine 3 receptor antagonist; CINV=chemotherapy-induced nausea and vomiting; EHR=electronic health record; HEC=highly emetogenic chemotherapy; IV=intravenous; MEC=moderately emetogenic chemotherapy;
NK1 RA=neurokinin-1 receptor antagonist.
NK1 RA=neurokinin-1 receptor antagonist.