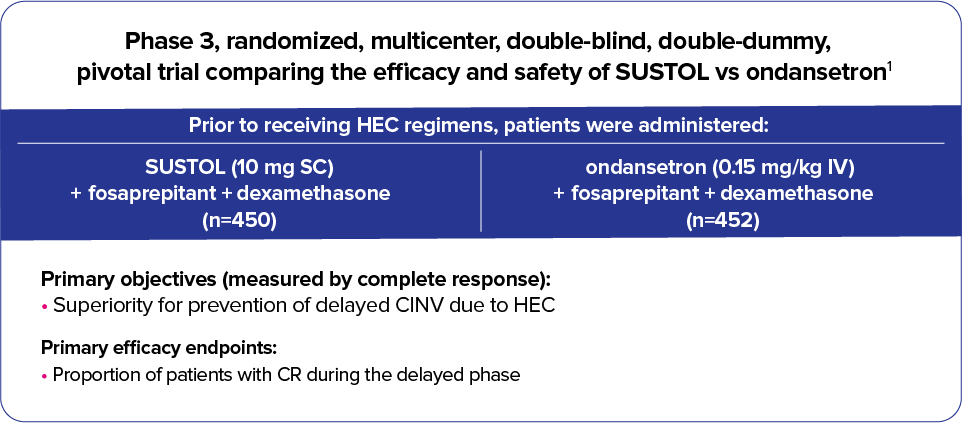
Trial 2 (MAGIC*)—SUSTOL is the only 5-HT3 RA to demonstrate superiority in a Phase 3,
3-drug vs 3-drug trial in HEC1
SUSTOL demonstrated superiority in the delayed phase of CINV vs ondansetron

- Modified Absorption of Granisetron In the Prevention of CINV.2
- CR was defined as no emetic episodes, including retching, and no use of rescue medications.1
- 2-sided hypothesis test. Significance level at α=0.05.1
- All patients were concomitantly administered intravenous dexamethasone.1
- SUSTOL demonstrated superiority vs ondansetron IV in the prevention of delayed CINV in a trial of over 900 patients receiving HEC regimens encompassing AC, cisplatin, and other regimens1
- A prespecified subgroup analysis of the cisplatin and non-cisplatin strata demonstrated consistent treatment benefit compared to the overall study population. However, the study was not powered to show statistical significance within subset analyses1
- Due to the number of cisplatin-treated patients in SUSTOL Phase 3 clinical trials (n=358; 26% of HEC-treated patients), efficacy in this population has not been established3-5
- Data shown are not included in the SUSTOL Prescribing Information. SUSTOL is not indicated in patients treated with cisplatin3
“This was the first published trial that compared a single dose of two different 5-HT3 antagonists when used in combination with dexamethasone and an NK1 RA.
“As a result, granisetron extended-release injection was the first FDA-approved 5-HT3 antagonist indicated for the prevention of delayed CINV associated with AC anticancer agents.”
– NCCN Guidelines®6
MAGIC was the first Phase 3 study to compare 3-drug vs 3-drug, guideline-recommended antiemetic regimens for the prevention of CINV in patients receiving HEC1,7
- 100% community-based setting1
- The most common chemotherapy regimens were AC-based regimens (65%) and cisplatin regimens (≥50 mg/m2) (28%)1
- Although palonosetron IV is commonly used, it has not shown superiority to ondansetron in a Phase 3 trial for delayed CINV due to HEC1
- Based on current NCCN Guidelines, palonosetron is not preferred over any other 5-HT3 RA for HEC treatment6
5-HT3 RA=5-hydroxytryptamine 3 receptor antagonist; AC=anthracycline/cyclophosphamide; CINV=chemotherapy-induced nausea and vomiting; CR=complete response; HEC=highly emetogenic chemotherapy; IV=intravenous; NCCN=National Comprehensive Cancer Network® (NCCN®); NK1 RA=neurokinin-1 receptor antagonist; SC=subcutaneous.