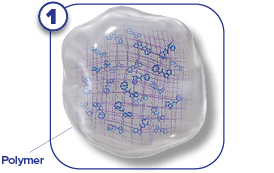
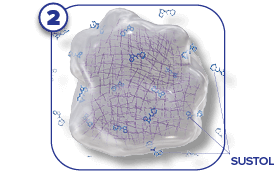
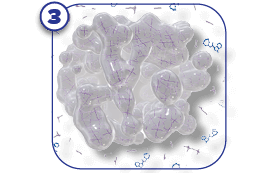
SUSTOL is a prescription medicine called an “antiemetic.” SUSTOL is used in adults to help prevent the nausea and vomiting that happens right away or later with certain anti-cancer medicines (chemotherapy).
Do not receive SUSTOL if you are allergic to granisetron, any of the ingredients in SUSTOL, or any other medicine of this type (5-HT3s) used to help prevent nausea and vomiting.
Injection site reactions may be serious and require medical care. Reactions include infections, bruising, swelling that is caused by blood that collects under the skin (hematoma), bleeding, pain, tenderness, and small bumps (nodules) at the injection site. Some reactions may occur 2 weeks or more after SUSTOL administration. Your risk of severe bruising and hematomas at the injection site is increased if you take a blood thinner medicine (anticoagulant or antiplatelet medicine). Get medical care right away if you have signs of an infection at the injection site or bleeding at the injection site that is severe or lasting more than 24 hours.
Stomach and intestinal problems, such as problems with bowel movement (constipation), may be serious. Tell your healthcare provider if you have constipation or your constipation worsens after you receive SUSTOL. Get medical care right away if you have pain or swelling in your stomach area (abdomen).
Serious allergic reactions have happened in people who received SUSTOL and who have had allergic reactions to other 5-HT3 medicines. These reactions may occur up to 7 days or longer following SUSTOL administration. Get emergency medical help right away if you have any signs or symptoms of a serious allergic reaction including hives, swollen face, breathing trouble, or chest pain.
Serotonin syndrome is a possible life-threatening problem that can happen when taking
5-HT3 medicines, especially when used with some medicines that treat depression and migraine headaches.
Before receiving SUSTOL, tell your healthcare provider about all of your medical conditions, including constipation, recent stomach-area (abdominal) surgery, kidney problems and about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Inform your doctor if you are pregnant or breastfeeding or plan to become pregnant or breastfeed.
The most common side effects of SUSTOL include: injection site reactions, constipation, fatigue, headache, diarrhea, stomach-area (abdominal) pain, trouble sleeping or falling asleep, indigestion, dizziness, weakness, and heartburn.
These are not all the possible side effects of SUSTOL. Ask your healthcare provider or pharmacist for more information. Call your doctor for medical advice about side effects.
You are encouraged to report side effects to the FDA at 1-800-FDA-1088 or . You may also report side effects to Heron at 1-844-437-6611.
SUSTOL is available by prescription only. For more information, please read the